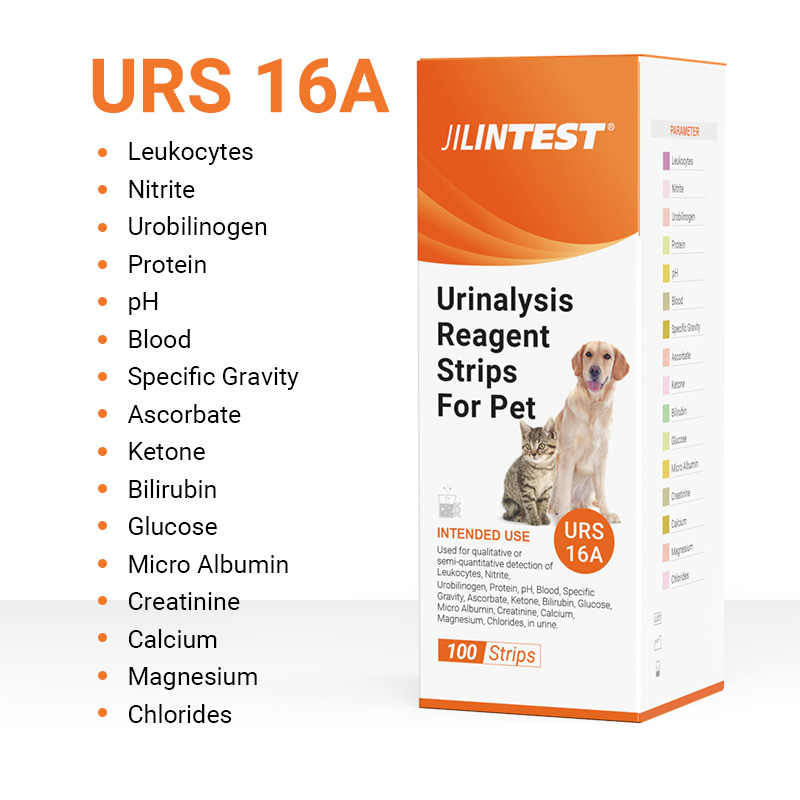
URS16A Urinalysis Reagent Test Strips
Brand :JILINTEST
Product origin :Changchun, Jilin, CN
Delivery time :3-4 weeks
Supply capacity :40k bottles of strips per month
16 parameter urine test strips for pets!
Using Scenario:
Veterinary Hospital
Home Daily Check for your little ones
| Product Name | JILINTEST URS16 Urinalysis Reagent Test Strips for Pets | |
| Shelf Life | 24 months | |
| Packaging | Brand/Neutral/Customized | |
| Testing Parameter | Leukocytes, Nitrite, Bilirubin, Protein, pH, Blood, Specific Gravity, Ketone, Urobilinogen, Glucose, Ascorbate, Micro Albumin, Creatinine, Calcium, Magnesium, Chloride | |
| Specification | 100 strips/bottle | |
| Certifications | FDA, ISO13485, etc. | |
Intended Use:
Used for qualitative or semi-quantitative detection of Leukocytes, Nitrite, Bilirubin, Protein, pH, Blood, Specific Gravity, Ketone, Urobilinogen, Glucose, Magnesium and Chloride in dogs, cats, rabbits, horses, cows, sheep, pigs, rats and other animal's urine solution.
Precautions:
1. Please read the manual carefullybefore use.
2. Store in a dry, cool and away fromdirect sunlight.
3. Use the test strips immediately after itopens.
4. Close the bottle tightly after removingstrips.
5. Do not remove the desiccant. Do nottouch the pads.
6. Only for one-time use.
7. Read the result under a good light.
8. Exposure to volatile substances(acidsbases, organic solvents, coal, etc.) orusing petroleum or coal heaters in thetesting environment may affect theaccuracy of the results.
9. lf there are doubts about the testresults and they are not consistentwith the expected results, pleaseconfirm whether the urinalysis reagentstrips has exceeded its validity periodand use quality control solution fortesting.
10. Please refer to the laboratory'smethods for handling biohazardousmaterials for handling used urinalysisreagent strips.
Sample requirements:
1. Collect fresh urine in a clean and dry container, do not centrifuge and do not addpreservatives.
2. Mix the urine well before testing.
3. At 22 C -28 C , urine should be tested within 2 hours without affecting the test results.4. lf the sample cannot be tested within 2 hours after collection, it can be refrigeratedand stored at a temperature of2 C-8 C.Glucose ,Leukocytes , blood . andcreatinine can be refrigerated and stored at a temperature of 2 C -8 ℃ for more than6 hours, resulting in inaccurate test results; Ketone and Ascorbic Acid are two itemsthat are stored in cold storage at a temperature of 2 C -8 C for more than 4 hours,and the test results are prone to inaccurate measurement values; Urobilinogen andbilirubin are prone to inaccurate measurements when stored in cold storage at atemperature of 2 C -8 ℃ for more than 2 hours.
Test Principle:
1. Leukocytes: the leukocyte esterase method is used. Neutrophil cytoplasm containsspecific esterase, this enzyme can act on the reagent block of indole acetic acid ester, sothat it produces indole phenol, the latter and the reaction of the color-forming substancesto form a purple condensate, the depth of its color is proportional to the number of cells.
2. Nitrite: the reaction depends on the reduction of nitrate to nitrite by gram-negativebacteria in urine. Nitrite can form red compounds with N,N-diethyl-p-phenylenediaminehydrochloride.
3. Urobilinogen: According to the principle of azo binding method. Urobilinogen is coupledwith solid blue B salt under strong acidic conditions to form cochineal pigment.
4. Protein: based on the principle of the dye-bound protein error method. Proteins bind tothe dye to form a complex to produce a color change, especially for albumin is moresensitive to the reaction than for globulin, hemoglobin, Benzoy's protein and mucin.
5. pH: Acid-base indicator method, which determines the pH value in the range of 5.0 to 8.5by means of a pH indicator.
6. Blood: Peroxidase method is used. Hemoglobin contains heme groups withperoxidase-ike activity, which can dehydrogenate tetramethylbenzidine, a coloress colorsource in the test strip reagent block, into colored compounds, and the color shade isproportional to the number of red blood cells.
7. Specific gravity: using the polyelectrolyte method, the principle of ion exchange betweenelectrolytes and polyelectrolytes in urine. In the presence of cations, the polyelectrolytehydrogen ions are released through the exchange, causing a color change in thebromothymol blue indicator, with the color changing from blue through blue-green andfinally to yellow.
8. Ascorbic acid: Reduction method is used. Vitamin C has l,2-en-diol reducing group,which can reduce the oxidized pink 2,6 dichloroindophenol dye to colorless2,6-dichloro-di-p-phenolamine in acidic conditions.
9. Ketone: sodium nitrosoferricvanide method is used. The reagent block mainly containssodium nitroso ferrocyanide, which can react with acetoacetic acid in urine under alkalineconditions to produce a purplish-red compound.
10. Bilirubin: According to the principle of azo-coupling method. In a strong acid medium.2,4-dichloroaniline reacts specifically with bilirubin and produces different colorscorresponding to the concentration of bilirubin.
11. Glucose: Glucose oxidase method is used. Glucose oxidase in the reagent block acts onglucose in urine to form glucuronic acid and hydrogen peroxide. Under the catalysis ofperoxidase, the color source is oxidized to produce colored compounds.
12. Microalbumin: According to the principle of the dye-bound protein error method,microalbumin binds to the dve to form a complex that produces a color change and issensitive to albumin.
13. Creatinine: creatinine and 3,5 dinitrobenzoic acid generate a complex, the color changeof the reaction ranges from yellow to green, and the color change is proportional to the
amount of creatinine.
14. Calcium: Based on the complexation of calcium ions with o-cresolphthalein phthaleincomplexone produces a color reaction, the shade of color is directly proportional to theconcentration of calcium ions.
15. Magnesium: Magnesium ions react with calcium magnesium indicators to form a purplecomplex, and the depth of color is proportional to the content of magnesium ions. Addappropriate masking agents to eliminate interference from calcium ions.
16. Chlorides: Chlorides form precipitates with silver nitrate, and excess silver nitrate reactswith chromates to form red compounds.
Limitations:
1. The results of urine analysis test strips cannot be used as a single diagnosticbasis for diseases.
2. This product can only be used for testing urine samples and cannot be used fortesting other body fluid samples.
3. The detection limit depends on several factors: variability in color recognition.specific gravity, pH value, and changes in lighting conditions during visualinspection.
4. The testing results of this product are semi-quantitative; The highest concentrationin each individual item measured by this product is likely to be greater than thisconcentration in the sample. lt is recommended to dilute the sample with purewater before testing.
5. Due to the variability of urine samples and reading results, there is a certaindeviation between the values of analytes detected and the actual values.
Jilin Test Bio-Electron Co., Ltd.








Factory. We speicalize in producing water test strips for years....more
-
Download
- JILINTEST Catalog.pdf